Gene-ious at Work: Editing the Future for Patients with Sickle Cell Disease By Lucy Williams, PhD…

BSGCT Researcher Spotlight by Amelia Vale
The BSGCT Conference celebrated the path of research that led to today’s ground-breaking treatments.
As scientists from around the world descended on London last month to discuss monumental advances in gene and cell therapy research, it is befitting to reflect on one of the field’s greatest milestones to date: Strimvelis
Developed by GlaxoSmithKline and the San Raffaele Telethon Institute for Gene Therapy (TIGET), Strimvelis treats children with the fatal immune disorder ADA-SCID (adenosine-deaminase deficient severe combined immunodeficiency). ADA-SCID is part of the SCID family of disorders, which is widely known as bubble baby disease because those affected need to be kept in bubble-like isolation to survive.
Yet, after just one dose of Strimvelis, children who would rarely make it to their first birthday are today leading completely normal lives. As a result, in 2016 Strimvelis became the first stem cell-based gene therapy to be licensed and this year it became the first of such treatments to be cleared for NHS funding by NICE.
Strimvelis is also, however, very much a product of gene therapy’s rocky road to success. But the tide has finally turned for this once beleaguered field; and as this year’s conference made clear, we can expect a wave of treatments spanning numerous diseases to follow. Today, this revolutionary technology is coming of age right before our eyes and four-year-old Margaux Moreels, from Belgium, is living proof of what it means for humanity.

Margaux Moreels
When Margaux was born in September 2013, it was only a matter of weeks before she was rushed to hospital with a lung infection. The next few months were touch and go but on New Year’s Day 2014, Margaux’s condition became critical. “It was terrifying, Margaux was having such difficulty breathing that we thought we were going to lose her. She was ambulanced to the university hospital in Leuven and taken straight to the emergency unit,” her mother, Marijike Geens, recalls.
Eventually, Margaux’s condition stabilised. But over the next few weeks the bad news piled up until her parents were left with a terrible diagnosis: Margaux had inherited a fatal immune condition so rare that it only affects around 15 children a year in Europe.
ADA-SCID is caused by mutations in the ADA gene that produce the enzyme adenosine deaminase, which is necessary for our immune system to function properly. This enzyme’s job is to turn the molecule, deoxyadenosine, which is toxic to immune system white blood cells, into the harmless molecule deoxyinosine. Without the enzyme functioning properly, the toxic molecules build up and kill off the white blood cells. This leaves the body defenceless against the slightest germ.
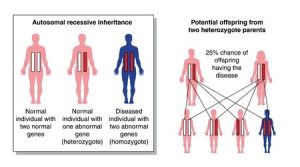
Margaux’s parents were shocked to discover that they were both carriers of this mutated gene. But as well as having a faulty ADA gene they had both inherited a normal copy, which stepped in to spare them of ADA-SCID. Unfortunately for Margaux, both of her parents’ faulty genes were passed on to her: there seemed to be no escaping this dreadful disease. (See autosomal recessive inheritance diagram.)
To make matters worse, it appeared that the only treatment for ADA-SCID was a stem cell transplant, but, as is the case with many patients, it was proving to be hard to find a matched donor. With every passing day Margaux’s life was hanging in the balance whereby exposure to the slightest virus, bacteria or fungus could have killed her. “At first I was scared of washing her without the nurse’s help. Even though we were in a completely sterilised room, I was worried I would do something wrong and put her life in danger,” says Marijike.
When Margaux’s situation seemed nigh-on impossible, she was suddenly thrown a life-line: ground-breaking gene therapy for ADA-SCID was being conducted in Milan. The treatment, they were told, would provide a corrected copy of that gene. “We had never heard of gene therapy before. It was very frightening but when were told how successful it had been for other patients, we jumped at the opportunity,” Hein Moreels, Margaux’s father, says.
A decade-long study published in the journal Blood in May 2016 1 reported that all 18 children who received the retroviral gene therapy in Milan were alive, and the majority were assessed to be “normal and fully active” by the medical performance index, Lansky. Later that year the data of four patients on an expanded access programme was released and more recently five patients have received Strimvelis: all are alive and well today.
Although Strimvelis costs a whopping £531,000, one dose is expected to last a life-time. The first patients to be treated on the clinical trial are now adults, a fact that swayed NICE to recommend Strimvelis for NHS funding. This was also the first time that NICE had applied its higher cost effectiveness limits for a rare disease. This new price threshold reflects the health benefits such treatments provide; raising the upper limit of funding to £300,000 per year of good quality of life for the patient. And for patients like Margaux, the benefits are priceless. Therefore, parents will do whatever it takes to get their child to Milan, the only place where Strimvelis is currently available.
As it was considered too risky for Margaux to fly to Italy, the family were advised to drive across Europe in a disinfected and sealed car. They were given an official letter to slide through the window at the border control that explained their situation. An infection acquired on route could have excluded Margaux from the therapy, so caution became the default mode.
On arrival in Milan, Margaux was assessed to be a good candidate for gene therapy so her physician, Professor Alessandro Aiuti, explained exactly what it would involve.
“Each patient undergoes a five-day treatment that includes cell withdrawal surgery, followed by chemotherapy while we correct their cells in the lab, and finally we reinfuse the corrected cells. After gene therapy, each baby spends at least a month in isolation until they have developed enough immunity to become a day patient.”
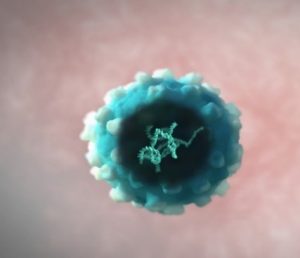
Illustration of the virus containing the corrected gene
To correct the cells, Prof Aiuti takes the DNA letters of a healthy ADA gene and puts them into a virus. As naked DNA cannot be directly injected into humans, viruses have been chosen because they naturally take their DNA into human cells when infecting them. After some nifty genetic engineering to remove their dangerous properties, viruses make the perfect delivery vehicle for the corrected cells.
“It is crucial for the treatment’s success that we use a patient’s blood stem cells,” Prof Aiuti emphasises. “These are the mother cells of all blood cells, which are found in the bone marrow. By getting the corrected gene into these cells, they become like factories of cells continuously self-renewing and churning out corrected cells for the patient’s entire life,” he adds.
The team in Milan were among the first scientists to learn how to engineer these all-important stem cells. This proved to be a real turning point for the field. But this was no overnight breakthrough, the lessons had been learned from decades of research from around the globe.

The San Raffaele Telethon Institute for Gene Therapy in Milan. Credit: Matt Lynch
The first official gene therapy on a patient happened back in 1990 and it was also designed to treat ADA-SCID. When the pioneers, Dr Michael Blaese and Dr French Anderson, treated four-year-old Ashanthi DeSilva at the National Institute of Health in America, the scientific landscape was very different. Yet, their early-day technique was actually surprisingly similar. They opted for the same delivery vehicle to correct the faulty cells: known as a retroviral vector in medical parlance because they use a retrovirus. However, the American team could only correct one type of blood cell – a T-cell that circulates through the blood system – and unfortunately, that T-cell would eventually die off.
Ashanthi receiving gene therapy in 1990
“We knew that stem cells were our best bet but the science just wasn’t there yet,” Dr Blaese recalls. “At one point about twenty-five percent of the target blood cells circulating inside Ashanthi had the corrected gene in them. 2Even though they gradually disappeared, a surprisingly large proportion of the cells that went in, were still there over 12 years later. 3 At the time, no one had any concept that these cells would live so many years,” he adds.

Dr Blaese at the Immune Deficiency Foundation 2013 National Conference. Credit: IDF
Today, Ashanthi does not produce much of the missing enzyme by herself, so she needs regular enzyme replacement therapy (ERT) and eight-hour immune-boosting infusions. Nevertheless, she holds down a job as a Magazine Editor and enjoys occasional trips abroad with her husband. “I am extremely grateful for my treatment,” Ashanthi says. “At the time, I could barely fight off infections on my own. I don’t think that I would have lived long without it. Those doctors saved my life, for sure,” Ashanthi emphasises. “It is truly wonderful how the scientists have perfected this gene therapy and to have been a very small part of it, is so gratifying. It’s amazing that today’s patients are doing so well – a whole world of opportunities is open to them.”
A world that awaits Margaux, simply because scientists mastered this technique using stem cells. A group at TIGET led by Prof Claudio Bordignon, who had been embedded in the early American research, were among those at the forefront of this science.
“TIGET had committed nearly a million euros to developing gene therapies for rare diseases so it was the perfect place to continue my studies,” Prof Bordignon recalls. “They didn’t have any experience with ADA-SCID but they had two researchers, Giuliana Ferrari and Fulvio Mavilio, who knew a lot about vectors,” he adds. Like two sides of a coin coming together, this led to a wealth of experience.
“Success didn’t happen overnight, many improvements over time made it possible,” according to Prof Bordignon. “We figured out the optimal cocktail for culturing the cells and we chose a retrovirus vector made by Ferrari that helped to produce the greatest amount of adenosine deaminase. So that was crucial, the more enzyme the better. And finally, in 1992 we treated patients with gene corrected stem cells,” he adds. 4
In the first clinical trials eight patients were treated with engineered blood stem cells in Europe and America. 4,5,6 But it wasn’t until the new millennium that this technology started to work well in patients. 7,8,9 Much of this success was down to administering a mild chemotherapy conditioning treatment to make space for the gene corrected cells in the bone marrow and stopping the patients’ ERT prior to the therapy to exploit the selective advantage of these cells.
However, it wasn’t long before tragedy ensued; over the next few years retroviral vector therapies caused nine cases of leukaemia (including one death) and three cases of bone marrow disease. Several international trials that were treating patients for immune disorders were affected, including at Great Ormond Street Hospital (GOSH) in London where one patient developed leukaemia.
“It was very disappointing. As well as being devastating for the families, this was a major setback for the whole field,” says Prof Bobby Gaspar, the Principle Trial Investigator at GOSH. “Our efforts went into designing and developing safer, better vectors that are now being realised in successful clinical trials such as ours,” he adds.
The tragedy forced the field to focus their sights on the lentiviral vector, which was already moving up the gene therapy ranks. Today, lentiviral vectors have all but taken over from their predecessor because they are both safer and more effective at modifying blood stem cells. As a result, they are being used in gene therapies to treat a wide range of diseases worldwide. But at the time of the cancer scare, they simply weren’t ready for TIGET’s trial so the team wasn’t prepared to ditch their lead player. On inspection, they saw that the retrovirus could be a bit hit and miss. Although it was a very effective delivery vehicle, it sometimes parked itself beside cancer causing genes that triggered leukaemia. For some reason, which is still not totally understood, no ADA-SCID patients were ever affected and today more than 40 people have been safely treated.
“It was a sad story,” Prof Aiuti recalls. “After halting the trial, we carefully went back to our patients and looked really hard for clues as to whether this could happen to them and we didn’t find any. Without the gene therapy patients were facing certain death so, after a period, we continued to treat them with very close monitoring, and this is what we are still doing today after many years,” he says.
Fortunately for patients with ADA-SCID, the success stories from this gene therapy kept coming. In 2010, the pharma giant GSK recognised the enormity of this breakthrough and partnered with TIGET to bring this product to market that much sooner. As GSK decided to sell off its rare disease unit last summer, Orchard Therapeutics have since taken up the reigns. Strimvelis and six other gene therapies developed at TIGET will join Orchard Therapeutics’ pipeline, which is already treating children with rare conditions, like Margaux, on both sides of the Atlantic.
“As the week of gene therapy approached, we were worried whether Margaux would be strong enough to endure it all,” Marijike recalls. “The most difficult part was the day of the cell withdrawal surgery. The anaesthetist promised to take care of Margaux as if she was her own daughter, which was so comforting. But as she drove Margaux away on the gurney, my heart just sank.”
The treatment, however, could not have gone any better. The surgery team managed to take plenty of cells, which quickly made their way over to the manufacturing site, MolMed, where Prof Bordignon and his team corrected them. On September 5th, almost a year after Margaux was born, she received the cells that have given her a completely new life.

Margaux at a check-up with Prof Aiuti and her brother, Mathieu
Today, a bubbly Margaux delights in all the thrilling adventures at kindergarten. She has developed a healthy immune system, has responded well to her first vaccines and needs no medication whatsoever.
“I remember when we were able to take Margaux to the playground for the first time, she was so fascinated by all the children. She roared with laughter when we put her on the slide and she kept climbing up for another go,” Marijike remembers. “This autumn Margaux will start school with all the other children her age. After the first two years of her life were spent in isolation, I can’t tell you how much this means to us.”
References
1 Cicalese, MP et al. Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood. Published May 25, 2016. DOI10.1182/blood-2016-01-688226.
2 R. Michael Blaese, Kenneth W. Culver, A. Dusty Miller, Charles S. Carter, Thomas Fleisher, Mario Clerici, Gene Shearer, Lauren Chang, Yawen Chiang, Paul Tolstoshev, Jay J. Greenblatt, Steven A. Rosenberg, Harvey Klein, Melvin Berger, Craig A. Mullen, W. Jay Ramsey, Linda Muul, Richard A. Morgan, and W. French Anderson. T Lymphocyte-Directed Gene Therapy for ADA Deficiency SCID: Initial trial results after 4 years. Science 1995; 270:475-480.
3 Muul LM, Tuschong LM, Soenen SL, Jagadeesh GJ, Ramsey WJ, Long Z, Carter CS, Garabedian EK, AleyThe ne M, Brown M, Bernstein W, Schurman SH, Fleisher TA, Leitman SF, Dunbar CE, Blaese RM, Candotti F. Persistence and expression of the adenosine deaminase gene for twelve years and immune reaction to gene transfer components: Long-term results of the first clinical gene therapy trial. Blood 2003, 101:2563-2569.
4 Bordignon C, Notarangelo LD, Nobili N, Ferrari G, Casorati G, Panina P, Mazzolari E, Maggioni D, Rossi C, Servida P, Ugazio AG, Mavilio F Science. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA- immunodeficient patients. 1995 Oct 20;270(5235):470-5.
5 Hoogerbrugge P, van Beusechem V, Fischer A, et al. Bone marrow gene transfer in three patients with adenosine deaminase deficiency. Gene Ther. 1996;3(2):179–83.
6 Kohn DB, Weinberg KI, Nolta JA, et al. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat Med 1995;1:1017–23.
7 Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, Bousso P, Deist FL, Fischer A. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000 Apr 28;288(5466):669-72.
8 Dando J, Aiuti A, Deola S, et al. Optimisation of retroviral supernatant production conditions for the genetic modification of human CD34+ cells. J Gene Med 2001;3:219–227.
9 Aiuti A, Slavin S, Aker M, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science (80- ) 2002;296:2410–3.
e: amelia@factualeyes.com t: +44(0)207 486 7918 m: +44(0)7932733232 www.factualeyes.com



