By Leoma Bere A new and improved method of cell therapy has shown promising early…

Making gene and cell therapy possible: Viruses
So, we understand the problem. How do we fix it?
Author: Alexander T. Baker, Senior Scientist, Accession Therapeutics
Years of research have borne fruit, we have built an understanding of what causes a disease and, crucially, what needs to be fixed. Perhaps it’s mutations in the CFTR gene causing cystic fibrosis(1), a deletion in the SMN1 gene leading to spinal muscular atrophy(2), or knowing that Lymphoma can be treated by targeting a protein called CD19(3). Now the question becomes:
How do we use this information to treat the disease?
Traditional medical interventions often revolve around removing the cells which are causing the problem (e.g. surgical excision of a tumour) or using drugs to attack the diseases’ underlying cause. So where do we turn when we can’t remove or destroy the problem because, either, attacking the defective cells is too harmful to the patient or because the disease will return later and the treatment will no longer be effective(4)? Afterall, those affected by cystic fibrosis still need their lungs, even if they cause difficulties.
The field of cell and gene therapy is an attempt to answer this question. These therapies attempt to induce the body to correct the causes of these conditions. This might be by telling cells how to make correct versions of a defective protein, as is the case in gene therapy for spinal muscular atrophy, or by inducing immune cells to recognize cancer cells and remove them, as in CAR-T therapy for lymphoma.
Viruses are the unlikely heroes making most gene and cell therapies a reality. Many viruses have evolved to be specialists at entering human cells and delivering genetic material right where they want it to be. Normally this is with the goal of copying themselves and spreading, a process which can cause disease and has made them infamous. But virologists, answering the call from oncologists and geneticists of a way to deliver instructions to human cells, are harnessing viruses as genetic messengers, making it possible, for the first time, to overcome the limitations of our genetic code and induce our bodies to fight off invading cancer cells, or make functional proteins critical for healthy organs.

Lentivirus (Left) and Adeno-associated Virus (Right) can carry DNA into human cells and stitch it into the cells genomes. Lentivirus is used to teach T-cells how to make new proteins so they can recognise and attack cancer cells. AAV is used to provide the body with new, functional, version of genes which those affect by genetic disease might lack so the cells can function in a healthy way.
Each virus has its own unique abilities and limitations. Perhaps you need to induce immune cells for the removal of cancerous B-cells? Lentivirus treatments are based on viruses especially evolved to enter and deliver genetic information to T-cells. In the approved cell therapy, Kymriah, this ability is used to induce T-cells to recognize and destroy cancer(5). Zolgensma is an approved gene therapy for spinal muscular atrophy (SMA) (6), a disease where motor-neurons die-off leading to progressive muscle loss and paralysis in early childhood. Zolgensma uses the ability of adeno-associated virus type 9 (AAV-9) to travel inside motor neurons and deliver the information they need to survive. Or perhaps a cancer has advanced, and surgery isn’t an option. What if we could inject a virus which is only harmful to cancer cells, destroying the cancer but not healthy ones? This is the idea behind the Voyager-V1 oncolytic vesicular stomatitis virus (VSV) which is in Phase II clinical trials(7).
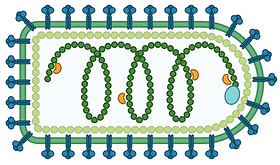
Vesicular Stomatitis Virus, VSV, is not normally found in humans as it can’t grow properly in healthy human cells. However, some of the genes which are broken in cancer cells which makes them cause disease are also the genes they need to fight off viruses. As a result VSV can often grow in, and destroy, cancer cells while leaving healthy cells alone. It is already being tested in the clinic and could, one day, be a new weapon against cancer.
Of course, it is not as simple to turn these viruses into effective therapies as imagining them, and other non-viral delivery approaches for gene and cell therapy are also being explored. The “viral delivery” success stories have taken decades of research, collaborations between scientists and clinicians of every discipline from fundamental virology to applied radiology. However, they have all begun with the recognition of a need, the repurposing of tools already in nature, and optimization through careful study and engineering. For example, many of the lentiviruses used today are the combination of parts taken from both HIV and VSV, with the virus genome carefully modified to prevent it from copying itself.
Scientists have only begun to scratch the surface of the different viruses which exist in nature. This is devastatingly apparent in the context of the current pandemic, where a lack of surveillance and hesitancy to act allowed a coronavirus subtype run amok. Further study of virologists, therefore, serves 2 purposes. The obvious reason is that it helps to prepare us to fight viral disease by preparing new anti-viral drugs and vaccines. Maybe less apparent, is that viruses represent an untapped cornucopia of tools just waiting to be harnessed as treatments for disease. Who knows what abilities viruses have already acquired and what problems they have already solved? If history is anything to go by, then we could be only one virus away from the cure to what was once incurable.
Alexander T. Baker
Senior Scientist,
Accession Therapeutics
References:
-
Basics of the CFTR Protein | Cystic Fibrosis Foundation. https://www.cff.org/research-clinical-trials/basics-cftr-protein.
-
What is SMA (Spinal Muscular Atrophy)? | SMN1 & SMN2 Genes. https://www.togetherinsma.com/en_us/home/introduction-to-sma/www.togetherinsma.com/en_us/home/introduction-to-sma/smn1-gene.html.
-
Makita, S. & Tobinai, K. Antibody therapy targeting CD19 for B-cell non-Hodgkin’s lymphoma. Annals of Oncology 29, 1086–1089 (2018).
-
Housman, G. et al. Drug Resistance in Cancer: An Overview. Cancers (Basel) 6, 1769–1792 (2014).
-
EMA. Kymriah. European Medicines Agency https://www.ema.europa.eu/en/medicines/human/EPAR/kymriah (2018).
-
EMA. Zolgensma. European Medicines Agency https://www.ema.europa.eu/en/medicines/human/EPAR/zolgensma (2020).
-
Sznol, M. et al. Phase II trial of Voyager-V1 (vesicular stomatitis virus expressing human IFNβ and NIS, VV1), in combination with cemiplimab (C) in patients with NSCLC, melanoma, HCC or endometrial carcinoma. JCO 38, TPS3161–TPS3161 (2020).


